Nguyen, Q. N. N.; Tantillo, D. J. “Using quantum chemical computations of NMR chemical shifts to assign relative configurations of terpenes from an engineered Streptomyces host,” J. Antibiotics 2016, 69, 534–540
Khokhar, S.; Pierens, G. K.; Hooper, J. N. A.; Ekins, M. G.; Feng, Y.; Rohan A. Davis, R. A. “Rhodocomatulin-Type Anthraquinones from the Australian Marine Invertebrates Clathria hirsuta and Comatula rotalaria,” J. Nat. Prod., 2016, 79, 946–953
Contributed by Steven Bachrach
Reposted from Computational Organic Chemistry with permission
 '
'
This work is licensed under a Creative Commons Attribution-NoDerivs 3.0 Unported License.
Khokhar, S.; Pierens, G. K.; Hooper, J. N. A.; Ekins, M. G.; Feng, Y.; Rohan A. Davis, R. A. “Rhodocomatulin-Type Anthraquinones from the Australian Marine Invertebrates Clathria hirsuta and Comatula rotalaria,” J. Nat. Prod., 2016, 79, 946–953
Contributed by Steven Bachrach
Reposted from Computational Organic Chemistry with permission
Use of computed NMR chemical shifts in structure determination is really growing fast. Presented here are a couple of recent examples.
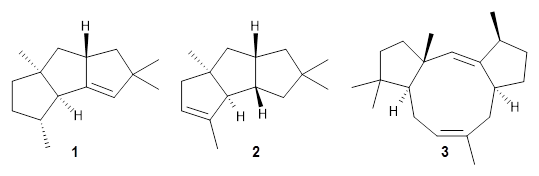
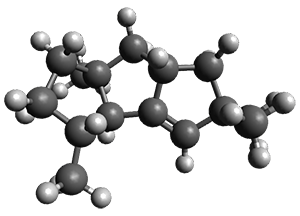
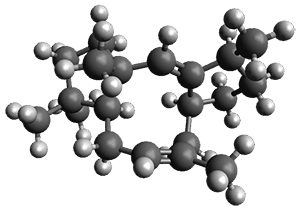
Nguyen and Tantillo used computed chemical shifts with the DP4 analysis to identify the structure of three terpenes 1-3.1 They optimized the geometries of all of the diastereomers of each compound, along with multiple conformations of each diastereomer, at B3LYP/6-31+G(d,p) and then computed the chemical shifts at SMD(CHCl3)–mPW1PW91/6-311+G(2d,p). The chemical shifts were Boltzmann weighted including all conformations within 3 kcal mol-1 of the lowest energy structure.

For 1, the DP4 analysis using just the proton shifts predicted a different isomer than using the carbon shifts, but when combined, DP4 predicted the structure, with 98.8% confidence, shown in the scheme above, and in Figure 1. For 2, the combined proton and carbon shift analysis with DP4 indicated a 100% confidence of the structure shown in the scheme and Figure 1. Lastly, for 3, which is more complicated due to the conformations of the 9-member ring, DP4 predicts with 100% confidence the structure shown in the scheme and Figure 1.
1
|
2
|
3
|
Figure 1. Optimized geometries of 1-3.
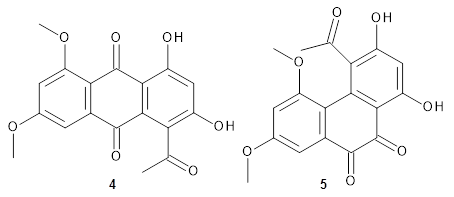
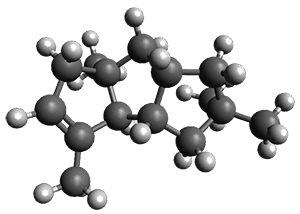
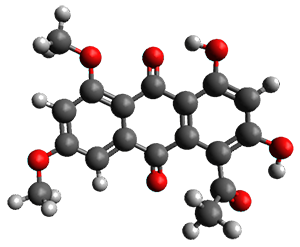
Feng, Davis and coworkers have examined a series of anthroquionones from Australian marine sponges.2The structure of one compound was a choice of two options: 4 or 5. Initial geometries were obtain by molecular mechanics and the low energy isomers were then reoptimized at B3LYP/6-31+G(d,p). The chemical shifts were computed using PCM/MPW1PW91/6-311+G(2d,p). Application of the DP4 method indicate the structure to be 4 with a 100% confidence level. The lowest energy conformer of 4 is shown in Figure 2.

Figure 2. Optimized geometry of 4.
References
1) Nguyen, Q. N. N.; Tantillo, D. J. “Using quantum chemical computations of NMR chemical shifts to assign relative configurations of terpenes from an engineered Streptomyces host,” J. Antibiotics 2016, 69, 534–540, DOI: 10.1038/ja.2016.51.
2) Khokhar, S.; Pierens, G. K.; Hooper, J. N. A.; Ekins, M. G.; Feng, Y.; Rohan A. Davis, R. A. “Rhodocomatulin-Type Anthraquinones from the Australian Marine Invertebrates Clathria hirsuta andComatula rotalaria,” J. Nat. Prod., 2016, 79, 946–953, DOI: 10.1021/acs.jnatprod.5b01029.
InChIs
1: InChI=1S/C15H24/c1-10-5-6-15(4)8-11-7-14(2,3)9-12(11)13(10)15/h9-11,13H,5-8H2,1-4H3/t10-,11+,13-,15+/m1/s1
InChIKey=KVSCZIPUFBVHBM-OICBVUGWSA-N
InChIKey=KVSCZIPUFBVHBM-OICBVUGWSA-N
2: InChI=1S/C15H24/c1-10-5-6-15(4)8-11-7-14(2,3)9-12(11)13(10)15/h5,11-13H,6-9H2,1-4H3/t11-,12-,13+,15-/m0/s1
InChIKey=ZLYGJLHCPYVGDA-XPCVCDNBSA-N
InChIKey=ZLYGJLHCPYVGDA-XPCVCDNBSA-N
3: InChI=1S/C20H32/c1-14-6-9-18-19(3,4)10-11-20(18,5)13-17-15(2)7-8-16(17)12-14/h6,13,15-16,18H,7-12H2,1-5H3/b14-6-,17-13-/t15-,16-,18-,20+/m0/s1
InChIKey=JZGOFJIAHJJJDK-ICZJPRMTSA-N
InChIKey=JZGOFJIAHJJJDK-ICZJPRMTSA-N
4: InChI=1S/C18H14O7/c1-7(19)13-10(20)6-11(21)15-16(13)17(22)9-4-8(24-2)5-12(25-3)14(9)18(15)23/h4-6,20-21H,1-3H3
InChIKey=MPQMZEXRJVMYBT-UHFFFAOYSA-N
InChIKey=MPQMZEXRJVMYBT-UHFFFAOYSA-N
5: InChI=1S/C18H14O7/c1-7(19)13-10(20)6-11(21)15-16(13)14-9(17(22)18(15)23)4-8(24-2)5-12(14)25-3/h4-6,20-21H,1-3H3
InChIKey=WIKIUXNPFURKNF-UHFFFAOYSA-N
InChIKey=WIKIUXNPFURKNF-UHFFFAOYSA-N
 '
'This work is licensed under a Creative Commons Attribution-NoDerivs 3.0 Unported License.
No comments:
Post a Comment