Hannes Kneiding, Ainara Nova, David Balcells (2023)
Highlighted by Jan Jensen
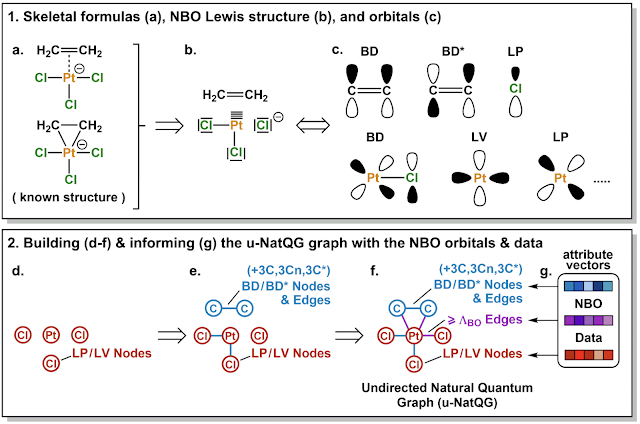
The authors show that an NBO analysis can be used to identify the charges (as well as their coordination mode) of individual ligands in TM-complexes. This is a key property needed to properly characterise the ligands and, thus, the complex as a whole. They have manually checked the approach for 500 compounds and finds that it gives reasonable results in 95% of the cases. That number drops to 92% if coordination mode is also considered. They provide these, and many other, properties of 30K ligands extracted from the CSD.
The NBO analysis is based on PBE/TZV//PBE/DZV calculations, which are a bit costly, but it will be interesting to see whether lower theories (e.g. DZV//xTB) give similar results.
Based on this knowledge the authors build a data set of 1.37B square-planar Pd compounds and compute their polarizability and HOMO-LUMO gap. They then search this space for molecules with both large polarizabilities and HOMO-LUMO gaps using a genetical algorithm that optimises the Pareto front, and show that optimum solutions can be found by considering only 1% if the entire space. The GA code is not available yet, but should be released soon.

This work is licensed under a Creative Commons Attribution 4.0 International License.