Feng, X.; Gu, J.; Chen, Q.; Lii, J.-H.; Allinger, N. L.; Xie, Y.; Schaefer, H. F. J. Chem. Theor. Comput. 2014, 10, 1511-1517
Contributed by Steven Bachrach.
Reposted from Computational Organic Chemistry with permission

This work is licensed under a Creative Commons Attribution-NoDerivs 3.0 Unported License.
Contributed by Steven Bachrach.
Reposted from Computational Organic Chemistry with permission
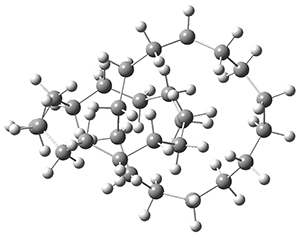
“How small can a catenane be?” This question is asked by Schaefer, Allinger and colleagues and answered (well, almost answered) using computations.1 Catenanes are linked rings. The catenanes examined here are two linked saturated hydrocarbon rings, each of the same size. The rings examined have 11 to 18 carbon atoms. The geometries were optimized with D2 symmetry, where either the closest approach between the two rings are two carbon atoms or the midpoint of two C-C bonds. The former turn out to be lower in energy. Geometries were optimized with MP2, B3LYP, BP86 or M06-2X with the DZP++ basis set. There is little geometric dependence on computational method. The optimized geometry of the catenane with 14 carbons is shown in Figure 1.
Figure 1. Optimized geometry of the 14-carbon catenane. (Be sure to click on this structure to view the molecule in 3-D; you will have to allow Jmol to download and run!)
To cut to the chase, as the rings get smaller they observe a lengthening of the C-C bonds at the intersection. With the 14-carbon catenane they observe a significant increase in the bond length near the intersection, suggesting a dramatic instability. This is also seen in the change in the energy per C as the rings get smaller; a large increase in energy per C is seen at the transition from 14 to 13 carbons. This all points toward the 14-carbon catenane as the smallest one that might be stable.
(I thank Prof. Schaefer and colleagues for providing me with the coordinates of the 14-carbon catenane.)
References
(1) Feng, X.; Gu, J.; Chen, Q.; Lii, J.-H.; Allinger, N. L.; Xie, Y.; Schaefer, H. F. "How Small Can a Catenane Be?," J. Chem. Theor. Comput. 2014, 10, 1511-1517, DOI: 10.1021/ct400926p

This work is licensed under a Creative Commons Attribution-NoDerivs 3.0 Unported License.
No comments:
Post a Comment