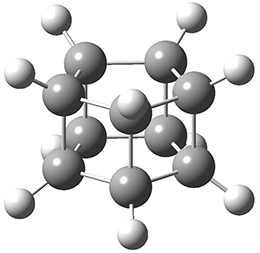
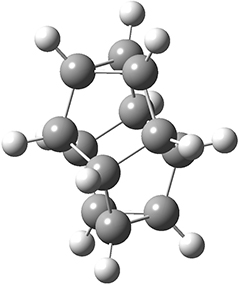
Paul M. Zimmerman (2015)
Contributed by Jan Jensen
This methodology takes a single structure as input automatically finds related intermediates and transition states connecting them to map out entire reaction mechanisms. The methodology is the combination of two different methods.
A single elementary step search "that takes a single chemical structure and proposes new intermediates that could be one elementary step away". Here an "elementary step" is defined as "chemical rearrangements with no more than two connections breaking and two connections forming simultaneously, while maintaining the upper and lower limits for coordination number of each atom." The energy of these intermediates are then computed using DFT and the ones with sufficiently low energy are kept.
The Growing String Method is then used to find the transition state and reaction path connecting the intermediates to the input structure and reaction paths with sufficiently low barriers are kept. This method, as implemented in Q-Chem, is completely automated and appears to be very robust.
The procedure is then repeated using the remaining intermediates as input until no new intermediates are formed. The thermodynamic and kinetic screening helps insure that the number of intermediates do no grow out of control. Also, for larger systems one can define inactive atoms (e.g. non-reactive ligands) that are not considered when creating new intermediates.

This work is licensed under a Creative Commons Attribution 4.0
Contributed by Jan Jensen
This methodology takes a single structure as input automatically finds related intermediates and transition states connecting them to map out entire reaction mechanisms. The methodology is the combination of two different methods.
A single elementary step search "that takes a single chemical structure and proposes new intermediates that could be one elementary step away". Here an "elementary step" is defined as "chemical rearrangements with no more than two connections breaking and two connections forming simultaneously, while maintaining the upper and lower limits for coordination number of each atom." The energy of these intermediates are then computed using DFT and the ones with sufficiently low energy are kept.
The Growing String Method is then used to find the transition state and reaction path connecting the intermediates to the input structure and reaction paths with sufficiently low barriers are kept. This method, as implemented in Q-Chem, is completely automated and appears to be very robust.
The procedure is then repeated using the remaining intermediates as input until no new intermediates are formed. The thermodynamic and kinetic screening helps insure that the number of intermediates do no grow out of control. Also, for larger systems one can define inactive atoms (e.g. non-reactive ligands) that are not considered when creating new intermediates.

This work is licensed under a Creative Commons Attribution 4.0