Kostenko, A.; Tumanskii, B.; Kobayashi, Y.; Nakamoto, M.; Sekiguchi, A.; Apeloig, Y., Angew. Chem. Int. Ed. 2017, 56, 10183-10187
Contributed by Steven Bacharach
Reposted from Computational Organic Chemistry with permission
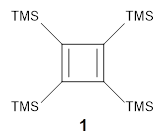
 '
'
This work is licensed under a Creative Commons Attribution-NoDerivs 3.0 Unported License.
Contributed by Steven Bacharach
Reposted from Computational Organic Chemistry with permission
Cyclobutadiene has long fascinated organic chemists. It is the 4e analogue of the 6e benzene molecule, yet it could hardly be more different. Despite nearly a century of effort, cyclobutadiene analogues were only first prepared in the 1970s, reflecting its strong antiaromatic character.
Per-trimethylsilylcyclobutadiene 1 offers opportunities to probe the properties of the cyclobutadiene ring as the bulky substituents diminish dimerization and polymerization of the reactive π-bonds. Kostenko and coworkers have now reported on the triplet state of 1.1 They observe three EPR signals of 1 at temperatures above 350 K, and these signals increase in area with increasing temperature. This is strong evidence for the existence of triplet 1 in equilibrium with the lower energy singlet. Using the variable temperature EPR spectra, the singlet triplet gap is 13.9 ± 0.8 kcal mol-1.

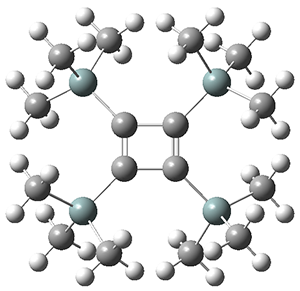
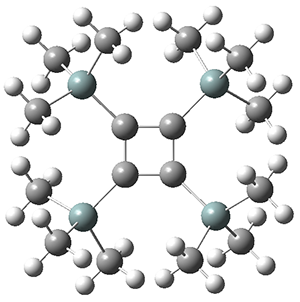
The structures of singlet and triplet 1 were optimized at B3LYP-D3/6-311+G(d,p) and shown in Figure 1. The singlet is the expected rectangle, with distinctly different C-C distance around the ring. The triplet is a square, with equivalent C-C distances. Since both the singlet and triplet states are likely to have multireference character, the energies of both states were obtained at RI-MRDDCI2-CASSCF(4,4)/def2-SVP//B3LYPD3/6-311+G(d,p) and give a singlet-triplet gap of 11.8 kcal mol-1, in quite reasonable agreement with experiment.
singlet
|
triplet
|
Figure 1. Optimized geometries of singlet and triplet 1.
References
1. Kostenko, A.; Tumanskii, B.; Kobayashi, Y.; Nakamoto, M.; Sekiguchi, A.; Apeloig, Y., "Spectroscopic Observation of the Triplet Diradical State of a Cyclobutadiene." Angew. Chem. Int. Ed. 2017, 56, 10183-10187, DOI: 10.1002/anie.201705228.
InChIs
1: InChI=1S/C16H36Si4/c1-17(2,3)13-14(18(4,5)6)16(20(10,11)12)15(13)19(7,8)9/h1-12H3
InChIkey=AYOHYRSQVCLGKR-UHFFFAOYSA-N
InChIkey=AYOHYRSQVCLGKR-UHFFFAOYSA-N
 '
'This work is licensed under a Creative Commons Attribution-NoDerivs 3.0 Unported License.
No comments:
Post a Comment