Kato, K.; Segawa, Y.; Scott, L. T.; Itami, K., Angew. Chem. Int. Ed. 2018, 57, 1337-1341
Contributed by Steven Bacharach
Contributed by Steven Bacharach
Reposted from Computational Organic Chemistry with permission
 '
'
This work is licensed under a Creative Commons Attribution-NoDerivs 3.0 Unported License.
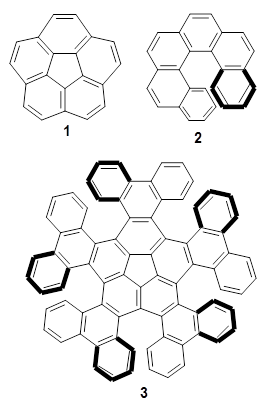
Corannulene 1 is an interesting aromatic compound because it is nonplanar, having a bowl shape. [6]helicene is an interesting aromatic compound because it is nonplanar, having the shape of a helix. Kato, Segawa, Scott and Itami have joined these together to synthesize the interesting quintuple helicene compound 3.1

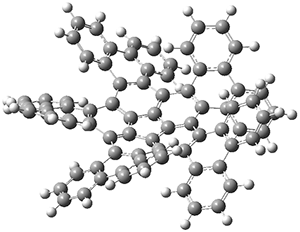
The optimized structure of 3 is shown in Figure 1. They utilized computations to corroborate two experimental findings. First, the NMR spectra of 3 shows a small number of signals indicating that the bowl inversion should be rapid. The molecule has C5 symmetry due to the bowl shape of the corannulene core. Rapid inversion makes the molecule effectively D5. (The inversion transition state is of D5 symmetry, and would be a nice quiz question for those looking for molecules of unusual point groups.) The B3LYP/6-31G(d) computed bowl inversion barrier is only 1.9 kcal mol-1, significantly less that the bowl inversion barrier of 1: 10.4 kcal mol-1. This reduction is partly due to the shallower bowl depth of 3 (0.572 Å in the x-ray structure, 0.325 Å in the computed structure) than in 1 (0.87 Å).

Figure 1. Optimized structure of 3.
Second, they took the enhanced MMMMM-isomer and heated it to obtain the thermodynamic properties for the inversion to the PPPPP-isomer. (The PPPPP-isomer is shown in the top scheme.) The experimental values are ΔH‡ = 36.8 kcal mol-1, ΔS‡ = 8.7 cal mol-1 K-1, and ΔG‡ = 34.2 kcal mol-1 at 298 K. They computed all of the stereoisomers of 3 along with the transition states connecting them. The largest barrier is found in going from MMMMM–3 to MMMMP–3 with a computed barrier of 34.5 kcal mol-1, in nice agreement with experiment.
References
1. Kato, K.; Segawa, Y.; Scott, L. T.; Itami, K., "A Quintuple [6]Helicene with a Corannulene Core as a C5-Symmetric Propeller-Shaped π-System." Angew. Chem. Int. Ed. 2018, 57, 1337-1341, DOI: 10.1002/anie.201711985.
InChIs
1: InChI=1S/C20H10/c1-2-12-5-6-14-9-10-15-8-7-13-4-3-11(1)16-17(12)19(14)20(15)18(13)16/h1-10H
InChIKey=VXRUJZQPKRBJKH-UHFFFAOYSA-N
InChIKey=VXRUJZQPKRBJKH-UHFFFAOYSA-N
2: InChI=1S/C26H16/c1-3-7-22-17(5-1)9-11-19-13-15-21-16-14-20-12-10-18-6-2-4-8-23(18)25(20)26(21)24(19)22/h1-16H
InChIKey=UOYPNWSDSPYOSN-UHFFFAOYSA-N
InChIKey=UOYPNWSDSPYOSN-UHFFFAOYSA-N
3: InChI=1S/C80H40/c1-11-31-51-41(21-1)42-22-2-12-32-52(42)62-61(51)71-63-53-33-13-3-23-43(53)44-24-4-14-34-54(44)64(63)73-67-57-37-17-7-27-47(57)48-28-8-18-38-58(48)68(67)75-70-60-40-20-10-30-50(60)49-29-9-19-39-59(49)69(70)74-66-56-36-16-6-26-46(56)45-25-5-15-35-55(45)65(66)72(62)77-76(71)78(73)80(75)79(74)77/h1-40H
InChIKey=XYUIBQJVZTYREY-UHFFFAOYSA-N
InChIKey=XYUIBQJVZTYREY-UHFFFAOYSA-N
 '
'This work is licensed under a Creative Commons Attribution-NoDerivs 3.0 Unported License.
No comments:
Post a Comment