Firouzi, R.; Shahbazian, S. ChemPhysChem 2016, 17, 51-54
Contributed by Steven Bachrach
Reposted from Computational Organic Chemistry with permission

This work is licensed under a Creative Commons Attribution-NoDerivs 3.0 Unported License.
Contributed by Steven Bachrach
Reposted from Computational Organic Chemistry with permission
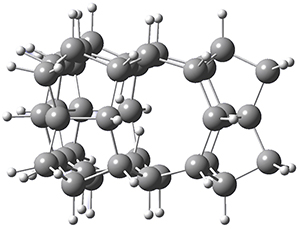
Setting the record for the shortest non-bonded H…H contact has become an active contest. Following on the report of a contact distance of only 1.47 Å that I blogged about here, Firouzi and Shahbazian propose a series of related cage molecules with C-H bonds pointed into their interior.1 The compounds were optimized with a variety of computational methods, and many of them have H…H distances well below that of the previous record. The shortest distance is found in 1, shown in Figure 1. The H…H distance in 1 is predicted to be less than 1.2 Å with a variety of density functionals and moderate basis sets.
1
|
Figure 1. Optimized geometry of 1 at ωB97X-D/cc-pVDZ.
References
(1) Firouzi, R.; Shahbazian, S. "Seeking Extremes in Molecular Design: To What Extent May Two “Non-Bonded” Hydrogen Atoms be Squeezed in a Hydrocarbon?," ChemPhysChem 2016, 17, 51-54, DOI:10.1002/cphc.201501002.
InChIs
1: InChI=1S/C41H44/c1-7-13-25-17-9-3-40-5-11-19(35(17)40)27-15-8-2-39(1,33(13)15)34-14(7)26-18-10-4-41-6-12-20(36(18)41)28(16(8)34)32(27)30-23(11)37(40)21(9)29(31(25)26)22(10)38(41)24(12)30/h7-38H,1-6H2
InChIKey=DMWSEJFKSWCSLI-UHFFFAOYSA-N
InChIKey=DMWSEJFKSWCSLI-UHFFFAOYSA-N

This work is licensed under a Creative Commons Attribution-NoDerivs 3.0 Unported License.
No comments:
Post a Comment