Poad, B. L. J.; Reed, N. D.; Hansen, C. S.; Trevitt, A. J.; Blanksby, S. J.; Mackay, E. G.; Sherburn, M. S.; Chan, B.; Radom, L., Chem. Sci. 2016, 7, 6245-6250
Contributed by Steven Bacharach
Reposted from Computational Organic Chemistry with permission
 '
'
This work is licensed under a Creative Commons Attribution-NoDerivs 3.0 Unported License.
Contributed by Steven Bacharach
Reposted from Computational Organic Chemistry with permission
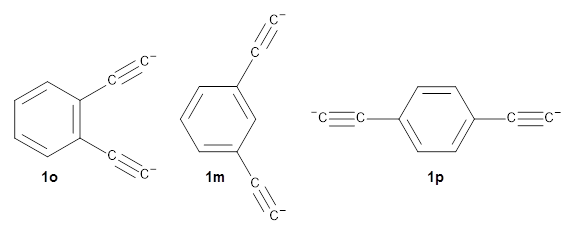
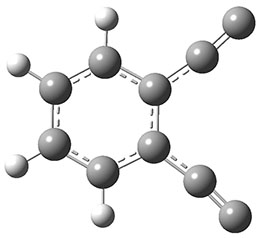
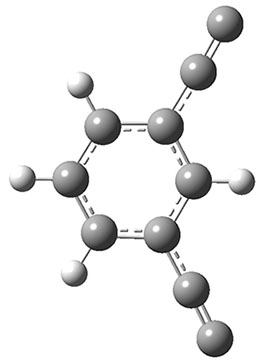
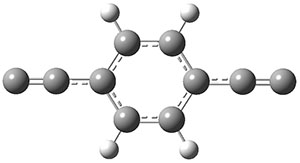
The new benchmark has been set for superbases. The previous record holder was LiO–, with a computed proton affinity of 424.9 kcal mol-1. A new study by Poad, et al., examines the dianions of the three isomeric phenyldiacetylides: 1o, 1m, and 1p.1 Their computed proton affinities (G4(MP2)-6X) are 440.6, 427.0, and 425.6 kcal mol-1, respectively. The optimized geometries of these dianions are shown in Figure 1.

1o
|
1m
|
1p
|
Figure 1. Optimized geometries of 1o, 1m, and 1p.
The authors also prepared these bases inside a mass spectrometer. All three deprotonate water, but do not deprotonate methane, though that might be a kinetic issue.
The authors speculate that 1o will be hard to beat as a base since loss of an electron is always a concern with small dianions.
References
1) Poad, B. L. J.; Reed, N. D.; Hansen, C. S.; Trevitt, A. J.; Blanksby, S. J.; Mackay, E. G.; Sherburn, M. S.; Chan, B.; Radom, L., "Preparation of an ion with the highest calculated proton affinity: ortho-diethynylbenzene dianion." Chem. Sci. 2016, 7, 6245-6250, DOI: 10.1039/C6SC01726F.
InChIs
1o: InChI=1S/C10H4/c1-3-9-7-5-6-8-10(9)4-2/h5-8H/q-2
InChIKey=RVSCTJNIQWGMPY-UHFFFAOYSA-N
InChIKey=RVSCTJNIQWGMPY-UHFFFAOYSA-N
1m: InChI=1S/C10H4/c1-3-9-6-5-7-10(4-2)8-9/h5-8H/q-2
InChIKey=ATCNFGGQUWGWOE-UHFFFAOYSA-N
InChIKey=ATCNFGGQUWGWOE-UHFFFAOYSA-N
1p: InChI=1S/C10H4/c1-3-9-5-7-10(4-2)8-6-9/h5-8H/q-2
InChIKey=GGQMWKMAMDPRPA-UHFFFAOYSA-N
InChIKey=GGQMWKMAMDPRPA-UHFFFAOYSA-N
 '
'This work is licensed under a Creative Commons Attribution-NoDerivs 3.0 Unported License.
No comments:
Post a Comment