Povie, G.; Segawa, Y.; Nishihara, T.; Miyauchi, Y.; Itami, K. Science 2017, 356, 172-175
Contributed by Steven Bacharach
Reposted from Computational Organic Chemistry with permission
 '
'
This work is licensed under a Creative Commons Attribution-NoDerivs 3.0 Unported License.
Contributed by Steven Bacharach
Reposted from Computational Organic Chemistry with permission
The synthesis of components of nanostructures (like fullerenes and nanotubes) has dramatically matured over the past few years. I have blogged about nanohoops before, and this post presents the recent work of the Itami group in preparing the nanobelt 1.1
 1 |
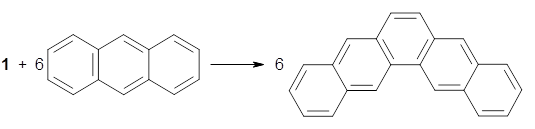
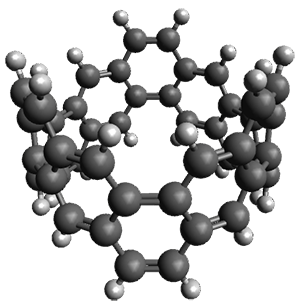
The synthesis is accomplished through a series of Wittig reactions with an aryl-aryl coupling to stitch together the final rings. The molecule is characterized by NMR and x-ray crystallography. The authors have also computed the structure of 1 at B3LYP/6-31G(d), shown in Figure 1. The computed C-C distances match up very well with the experimental distances. The strain energy of 1, presumably estimated by Reaction 1,2 is computed to be about 119 kcal mol-1.
1
|
Figure 1. B3LYP/6-31G(d) optimized structure of 1.
 |
Rxn 1
|
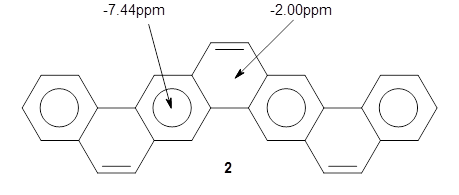
NICS(0) values were obtained at B3LYP/6-311+G(2d,p)//B3LYP/6-31G(d); the rings along the middle of the belt have values of -7.44ppm and are indicative of normal aromatic 6-member rings, while the other rings have values of -2.00ppm. This suggests the dominant resonance structure shown below:

References
1) Povie, G.; Segawa, Y.; Nishihara, T.; Miyauchi, Y.; Itami, K., "Synthesis of a carbon nanobelt." Science 2017, 356, 172-175, DOI: 10.1126/science.aam8158.
2) Segawa, Y.; Yagi, A.; Ito, H.; Itami, K., "A Theoretical Study on the Strain Energy of Carbon Nanobelts." Org. Letters 2016, 18, 1430-1433, DOI: 10.1021/acs.orglett.6b00365.
InChIs:
1: InChI=1S/C48H24/c1-2-26-14-40-28-5-6-31-20-44-32(19-42(31)40)9-10-34-24-48-36(23-46(34)44)12-11-35-21-45-33(22-47(35)48)8-7-30-17-41-29(18-43(30)45)4-3-27-15-37(39(26)16-28)25(1)13-38(27)41/h1-24H
InChIKey=KJWRWEMHJRCQKK-UHFFFAOYSA-N
InChIKey=KJWRWEMHJRCQKK-UHFFFAOYSA-N
 '
'This work is licensed under a Creative Commons Attribution-NoDerivs 3.0 Unported License.
No comments:
Post a Comment