A. Evidente, S. Superchi, A. Cimmino, G. Mazzeo, L. Mugnai, D. Rubiales, A. Andolfi, A. M. Villegas-Fernández, European Journal of Organic Chemistry 2011, 5564 (Paywall)
Contributed by Steven Bachrach.
Reposted from Computational Organic Chemistry with permission
It is striking to me that the absolute configuration of relatively simple compounds remains problematic even today. The structure of two naturally-occurring phytotoxic enantiomers 1, called regiolone and isosclerone, are finally definitively defined using a computational approach.
Isosclerone is the dextrorotatory isomer, while regiolone is the levorotatory isomer. The question though is which one is R and which one is S? Evidente and co-workers arbitrarily decided to compute the spectral properties of the S isomer.1 They located four low energy conformers at B3LYP/6-31G* and B3LYP/TZVP. (These conformers are not shown here as the authors did not deposit the coordinates. Reviewers and editors – please insist that this computational data be mandatory for publication!) The conformer relative energies, listed in Table 1, are dependent on the method, however, the two lowest energy structures will dominate the population and both will be present to a significant extent, regardless of which energy set is used. The optical rotation [α]D was computed at B3LYP/6-31G*//B3LYP/TZVP, and these too are listed in Table 1. The Boltzmann-weighted [α]D is 21.8. Even though the lowest energy conformer contributes a negative rotation, the much larger positive rotation due to the second-lowest energy conformer, along with the two other conformers, will dominate to dictate the OR value. This suggests that the enantiomers are (S)(+)-1 and (R)(-)-1. Computed ECD spectra confirm this assignment; the computed ECD of the (S) isomer is a near mirror image of the experimental ECD of the (-)-1 compound. Therefore, regiolone is (R)(-)-1 and isosclerone is (S)(+)-1.
(1) Evidente, A.; Superchi, S.; Cimmino, A.; Mazzeo, G.; Mugnai, L.; Rubiales, D.; Andolfi, A.; Villegas-Fernández, A. M., "Regiolone and Isosclerone, Two Enantiomeric Phytotoxic Naphthalenone Pentaketides: Computational Assignment of Absolute Configuration and Its Relationship with Phytotoxic Activity," Eur. J. Org. Chem., 2011, 5564-5570, DOI: 10.1002/ejoc.201100941

This work is licensed under a Creative Commons Attribution-NoDerivs 3.0 Unported License.
Contributed by Steven Bachrach.
Reposted from Computational Organic Chemistry with permission
It is striking to me that the absolute configuration of relatively simple compounds remains problematic even today. The structure of two naturally-occurring phytotoxic enantiomers 1, called regiolone and isosclerone, are finally definitively defined using a computational approach.
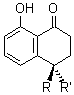
(R)-1: R = OH, R’ = H
(S)-1: R = H, R’ = OH
(S)-1: R = H, R’ = OH
Isosclerone is the dextrorotatory isomer, while regiolone is the levorotatory isomer. The question though is which one is R and which one is S? Evidente and co-workers arbitrarily decided to compute the spectral properties of the S isomer.1 They located four low energy conformers at B3LYP/6-31G* and B3LYP/TZVP. (These conformers are not shown here as the authors did not deposit the coordinates. Reviewers and editors – please insist that this computational data be mandatory for publication!) The conformer relative energies, listed in Table 1, are dependent on the method, however, the two lowest energy structures will dominate the population and both will be present to a significant extent, regardless of which energy set is used. The optical rotation [α]D was computed at B3LYP/6-31G*//B3LYP/TZVP, and these too are listed in Table 1. The Boltzmann-weighted [α]D is 21.8. Even though the lowest energy conformer contributes a negative rotation, the much larger positive rotation due to the second-lowest energy conformer, along with the two other conformers, will dominate to dictate the OR value. This suggests that the enantiomers are (S)(+)-1 and (R)(-)-1. Computed ECD spectra confirm this assignment; the computed ECD of the (S) isomer is a near mirror image of the experimental ECD of the (-)-1 compound. Therefore, regiolone is (R)(-)-1 and isosclerone is (S)(+)-1.
Table 1. Relative free energies (kcal mol-1) and [α]D of the conformers of (S)-1.a
| conformer | ΔG, 6-31G* | ΔG, TZVP | [α]Db |
| A | 0.43 | 0.0 | -17.50 |
| B | 0.0 | 0.32 | 67.92 |
| C | 1.21 | 1.03 | 95.72 |
| D | 1.84 | 1.48 | 17.72 |
aAll computations performed with B3LYP. bAt B3LYP/6-31G*//B3LYP/TZVP
References
(1) Evidente, A.; Superchi, S.; Cimmino, A.; Mazzeo, G.; Mugnai, L.; Rubiales, D.; Andolfi, A.; Villegas-Fernández, A. M., "Regiolone and Isosclerone, Two Enantiomeric Phytotoxic Naphthalenone Pentaketides: Computational Assignment of Absolute Configuration and Its Relationship with Phytotoxic Activity," Eur. J. Org. Chem., 2011, 5564-5570, DOI: 10.1002/ejoc.201100941

This work is licensed under a Creative Commons Attribution-NoDerivs 3.0 Unported License.
No comments:
Post a Comment