Scholz, F.; Himmel, D.; Heinemann, F. W.; Schleyer, P. v. R.; Meyer, K.; Krossing, I. Science 2013, 341, 62
Contributed by Steven Bachrach.
Reposted from Computational Organic Chemistry with permission

This work is licensed under a Creative Commons Attribution-NoDerivs 3.0 Unported License.
Contributed by Steven Bachrach.
Reposted from Computational Organic Chemistry with permission
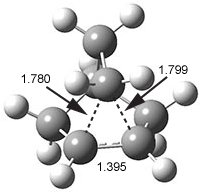
A long sought-after data point critical to the non-classical cation story has finally been obtained. The elusive x-ray crystal structure of a norbornyl cation was finally solved.1 The [C7H11]+[Al2Br7]- salt was crystallized in CH2Br2 at low temperature (40 K). This low temperature was needed to prohibit rotation of the norbornyl cation within the crystal (the cation is near spherical and so subject to relatively easy rotation within the crystal matrix) and hydride scrambling among the three carbons (C1, C2, and C6) involved in the non-classical cation structure.
The authors report a number of different structures, all very similar, depending on slight differences in the crystals used. However, the important features are consistent with all of the structures. The cation is definitely of the non-classical type (see Figure 1) with the basal C1-C2 bond length of 1.39 Å similar that in benzene and long non-classical C1-C6 and C2-C6 distances of 1.80 Å. These distances match very well with the MP2(FC)/def2-QZVPP optimized distances of 1.393 and 1.825 Å, respectively.
Figure 1. X-ray structure of norbornyl cation.
References
(1) Scholz, F.; Himmel, D.; Heinemann, F. W.; Schleyer, P. v. R.; Meyer, K.; Krossing, I. "Crystal Structure Determination of the Nonclassical 2-Norbornyl Cation," Science 2013, 341, 62-64, DOI:10.1126/science.1238849.

This work is licensed under a Creative Commons Attribution-NoDerivs 3.0 Unported License.
No comments:
Post a Comment