Yu, P.; Chen, T. Q.; Yang, Z.; He, C. Q.; Patel, A.; Lam, Y.-h.; Liu, C.-Y.; Houk, K. N., J. Am. Chem. Soc. 2017, 139 (24), 8251-8258
Contributed by Steven Bacharach
Reposted from Computational Organic Chemistry with permission
 '
'
This work is licensed under a Creative Commons Attribution-NoDerivs 3.0 Unported License.
Contributed by Steven Bacharach
Reposted from Computational Organic Chemistry with permission
Bispericyclic transition states arise when two pericyclic reactions merge to a common transition state. This leads to a potential energy surface with a bifurcation such that reactions that traverse this type of transition state will head towards two different products. The classic example is the dimerization of cyclopentadiene, involving two [4+2] Diels-Alder reactions. Unusual PESs are discussed in my book and in past blog posts.
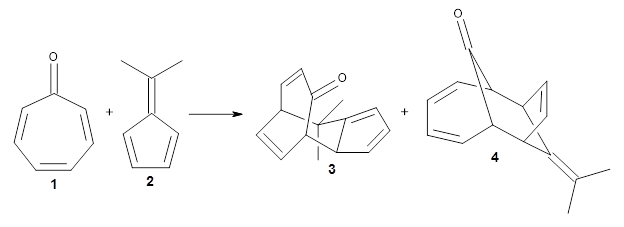
Houk and coworkers have now identified a bispericyclic transition state involving two [6+4] cycloadditions.1 Reaching back to work Houk pursued as a graduate student with Woodward for inspiration, these authors examined the reaction of tropone 1 with dimethylfulvene 2. Each moiety can act as the diene or triene component of a [6+4] allowed cycloaddition:

The product with fulvene 2 as the 6 π-e component and tropone as the 4 π-e component [6F+4T] is 3, while reversing their participation in the [6T+4F] cycloaddition leads to 4. A variety of [4+2] reactions are also possible. All of these reactions were investigated at PCM/M06-2X/6-311+G(d,p)//B3LYP-D3/6-31G(d). The reaction leading to 3 is exothermic by 3.0 kcal mol-1, while the reaction to 4 endothermic by 1.3 kcal mol-1.
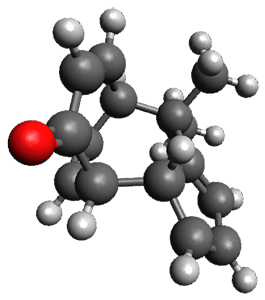
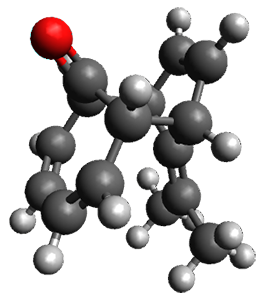
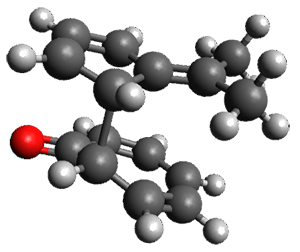
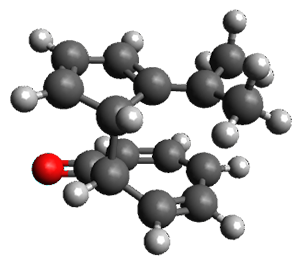
Interestingly, there is only one transition state that leads to both 3 and 4, the first known bispericyclic transition state for two conjoined [6+4] cycloadditions. The barrier is 27.9 kcal mol-1. The structures of the two products and the transition state leading to them are shown in Figure 1. 3 and 4 can interconvert through a Cope transition state, also shown in Figure 1, with a barrier of 26.3 kcal mol-1 (for 4 → 3).
3
|
4
|
TS [6+4]
|
TS Cope
|
Figure 1. B3LYP-D3/6-31G(d) optimized geometries.
Given that a single transition leads to two products, the product distribution is dependent on the molecular dynamics. A molecular dynamics simulation at B3LYP-D3/6-31G(d) with 117 trajectories indicates that 4 is formed 91% while 3 is formed only 9%. Once again, we are faced with the reality of much more complex reaction mechanisms/processes than simple models would suggest.
References
1) Yu, P.; Chen, T. Q.; Yang, Z.; He, C. Q.; Patel, A.; Lam, Y.-h.; Liu, C.-Y.; Houk, K. N., "Mechanisms and Origins of Periselectivity of the Ambimodal [6 + 4] Cycloadditions of Tropone to Dimethylfulvene." J. Am. Chem. Soc. 2017, 139 (24), 8251-8258, DOI: 10.1021/jacs.7b02966.
InChIs
1: InChI=1S/C7H6O/c8-7-5-3-1-2-4-6-7/h1-6H
InChIKey=QVWDCTQRORVHHT-UHFFFAOYSA-N
InChIKey=QVWDCTQRORVHHT-UHFFFAOYSA-N
2: InChI=1S/C8H10/c1-7(2)8-5-3-4-6-8/h3-6H,1-2H3
InChIKey=WXACXMWYHXOSIX-UHFFFAOYSA-N
InChIKey=WXACXMWYHXOSIX-UHFFFAOYSA-N
3:InChI=1S/C15H16O/c1-15(2)10-6-8-12(14(16)9-7-10)11-4-3-5-13(11)15/h3-12H,1-2H3
InChIKey=SEKRUGIZAIQCDA-UHFFFAOYSA-N
InChIKey=SEKRUGIZAIQCDA-UHFFFAOYSA-N
4: InChI=1S/C15H16O/c1-9(2)14-10-7-8-11(14)13-6-4-3-5-12(10)15(13)16/h3-8,10-13H,1-2H3
InChIKey=AQQAMUGJSGJKLC-UHFFFAOYSA-N
InChIKey=AQQAMUGJSGJKLC-UHFFFAOYSA-N
 '
'This work is licensed under a Creative Commons Attribution-NoDerivs 3.0 Unported License.
No comments:
Post a Comment